- +33 877 554 332
- info@veritasglobalcertification.com
ISO 13485: 2016
Medical Devices — Quality management systems
ISO 13485 Certification
ISO 13485: 2016 (Medical devices — Quality management systems — Requirements for regulatory purposes) is an ISO standard that represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices. Though it is tailored to the industry’s quality system expectations and regulatory requirements, an organization does not need to be actively manufacturing medical devices or their components to seek certification to this standard. ISO 13485: 2016 applies to design, development, production, installation and servicing of medical devices. Compliance is a measure of your ability to meet customer and legal requirements.
Why ISO 13485 Important For Business?
The main focus of QMS is to meet and deliver more than just customer needs. This focus will contribute greatly to organizational success in the long term. ISO 9001 provides the necessary infrastructure, procedures, processes and resources to help organizations monitor, improve and optimize performance to drive effectiveness, efficiency, customer service and product excellence. ISO 9001 certification helps organizations to deliver stakeholder engagement processes, organizational reputation, customer satisfaction and competitive benefits.
Benefits of ISO 13485
- Demonstrate that you produce safer and more effective medical devices
- Meet regulatory requirements and customer expectations
- Outline how to review and improve processes across your organisation
- Increase efficiency, cut costs and monitor supply chain performance
Increased access to more markets worldwide with certification
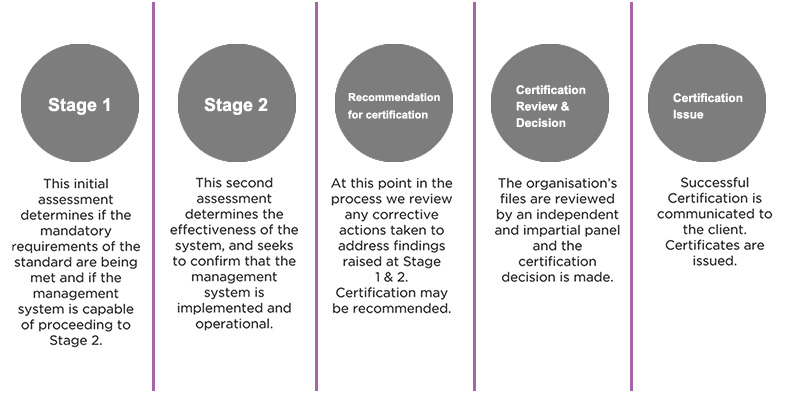
Certification Journey